Update hypercholesterolemie & ezetimibe
Literatuur - Phan BA, Dayspring TD, Toth PP - Vasc Health Risk Manag. 2012;8:415-27Ezetimibe therapy: mechanism of action and clinical update.
Phan BA, Dayspring TD, Toth PP.Vasc Health Risk Manag. 2012;8:415-27.
Achtergrond
Er is een duidelijk verband tussen verhoogde serum cholesterolwaarden en cardiovasculair risico [1,2]. Behandeling met statines verlaagt effectief LDL-C-waarden en vermindert ernstige cardiovasculaire gebeurtenissen [3-7]. Meer agressieve verlaging van de LDL-C kan het cardiovasculaire voordeel verhogen [8-10], vooral in hoog-risico patiënten met meerdere risicofactoren [11]. Om optimale LDL-C waarden te bereiken, is alleen statinetherapie wellicht niet voldoende. Bij deze patiënten wordt combinatie met andere cholesterolverlagende middelen aanbevolen, zoals de cholesterolabsorptie remmer ezetimibe. Het werkingsmechanisme, effecten op lipiden, en de veiligheid van behandeling met ezetimibe behandeling worden beschreven, evenals studies die van invloed kunnen zijn op het gebruik in de klinische praktijk.Samenvatting
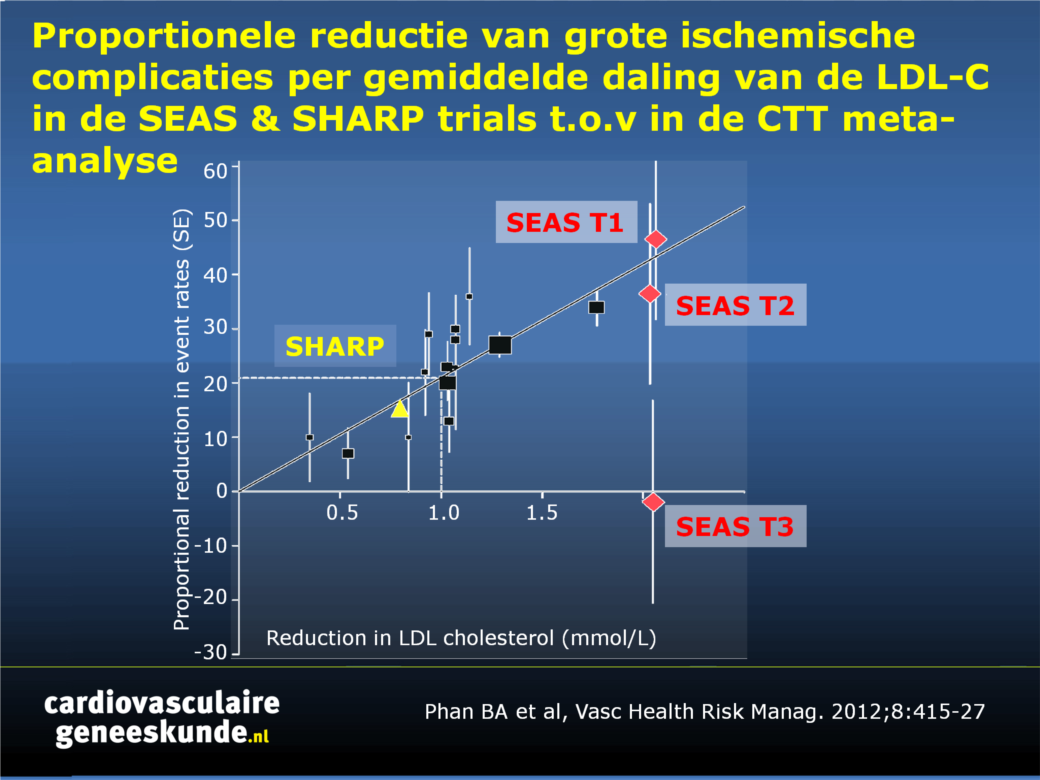
Ezetimibe remt intestinale absorptie van cholesterol door het selectief blokkeren van het Niemann-Pick C1-achtig eiwit (NPC1L1) in de jejunale brush border, een integraal onderdeel van de opname van intestinale lumen micellen in de enterocyt [12-15]. Het is, hetzij als monotherapie of in combinatie met statines, effectief in het verlagen van cholesterol in verschillende populaties, zoals insulineresistentie, FH en sitosterolemie.Zowel in de SANDS als de VYCTOR studie werd regressie van atherosclerose gerapporteerd [16-18]. De klinische werkzaamheid van behandeling met ezetimibe werd geëvalueerd in de SEAS studie [19] en in de SHARP studie [20], beide met ezetimibe plus simvastatine (figuur 1).
De ENHANCE [21] en ARBITER-6 studie [22] rapporteerden echter negatieve uitkomsten, hoewel beide studies methodologisch beperkt waren in hun vermogen om het voordeel van ezetimibe te evalueren. Daarom wordt met spanning uitgekeken naar de komende resultaten van de IMPROVE-IT-studie [23]. Deze resultaten kunnen het gebruik van ezetimibe in zeer hoog risico CHD populaties beter sturen.
Conclusie
Vooralsnog is ezetimibe een waardevolle aanvulling op statinetherapie bij de behandeling van hypercholesterolemie. Komende studieresultaten worden verwacht om het effect in de klinische praktijk beter te beoordelen.Figuur 1.
Proportionele reductie van grote ischemische complicaties per gemiddelde daling van de LDL-C (mmol / L) in de Simvastatine en Ezetimibe in Aortastenose (SEAS) trial (tertielen 1, 2, en 3 voor de ernst van de aortaklepstenose) ten opzichte van 14 gerandomiseerde trials in de Cholesterol Treatment Trialists meta-analyse.Klik op afbeelding om als PPT te downloaden
Referenties
1. Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. An Intern Med. 1979;90:85–91.2. Keys A, Menotti A, Aravanis C, et al. The seven countries study: 2,289 deaths in 15 years. Prev Med. 1984;13:141–154.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
4. [No authors listed]. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389.
5. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009.
6. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333: 1301–1307.
7. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279: 1615–1622.
8. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435.
9. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504.
10. Cannon CP, Steinberg BA, Murphy SA, Mega JL Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445.
11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732.
12. Bays H. Ezetimibe. Expert Opin Investig Drugs. 2002;11:1587–1604.
13. Altmann SW, Davis HR Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204.
14. Rosenblum SB, Huynh T, Afonso A, et al. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41: 973–980.
15. Davis HR Jr, Tershakovec AM, Tomassini JE, Musliner T. Intestinal sterol transporters and cholesterol absorption inhibition. Curr Opin Lipidol. 2011;22:467–478.
16. Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299:1678–1689.
17. Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205.
18. Meaney A, Ceballos G, Asbun J, et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J Clin Pharmacol. 2009;49:838–847.
19. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359: 1343–1356.
20. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192.
21. Kastelein JJP, Akdim F, Stroes ESG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. New Engl J Med. 2008;358: 1431–1443.
22. Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361: 2113–2122.
23. Cannon CP, Giugliano RP, Blazing MA, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–832.

Deel deze pagina met collega's en vrienden: